Caustic soda flakes and the methods of production

Caustic soda; Features along with a look into its sales market
Introduction to the alkaline compound of solid sodium hydroxide
Caustic soda flakes or caustic soda solids (sodium hydroxide) is a common and well-known alkaline compounds. This solid, commonly known as sodium hydroxide or caustic soda, is also sold in the market.
Solid sodium hydroxide (Caustic soda formula is NaOH) is in fact, a solid mass that has become flaky. This compound is matte or milky white.
This material is soluble in water and alcohol and is highly moisture-absorbent. It is classified as a corrosive chemical. Sodium hydroxide is obtained by evaporating the liquid caustic soda compound.
In the market, if you come across the following common names, the meaning is the same as alkaline substance:
Solid sodium hydroxide; Sodium hydroxide; Caustic soda; NaOH, solid NaOH; Natrium hydroxide; Sodium hydrate;
Solid caustic soda is used in the processes of synthesizing organic compounds, refining petroleum products, textile industries to produce silk and fabric bleaching, paper industry, and production of soap, aluminum metal, sodium, glass, etc.
read more: caustic soda uses
Contents
A brief look at the history of caustic soda (caustic soda meaning)
Sodium hydroxide was first developed by soap manufacturers. The method was performed according to the soap preparation instructions published in a book in Arabic.
The book mentioned was compiled by the King of Yemen named Muzaffar Yusuf bin Umar bin Ali bin Rasool. This method was considered one of the industrial inventions of the time.
In the method, it was suggested that water should be passed through the alkaline solution; therefore, the final product would be a combination of sodium hydroxide. The alkaline composition in this method was selected from crude sodium carbonate and quicklime or calcium oxide.
Interestingly, most soap manufacturers in European countries have based their production methods on these innovations and followed them.
In 1791, the French chemist and surgeon Nicolas Leblanc (1742-1806) invented a process for the mass production of sodium carbonate, the natural “soda ash” (= impure sodium carbonate obtained from the ashes of sodium-rich plants).
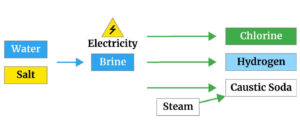
The new method was replaced by the previous unusual method. However, in the 20th century, the main method of producing solid sodium hydroxide or caustic soda is electrolysis of sodium chloride which will be elaborated on in the next section.
The method of Iranian caustic soda production
caustic soda production
What is caustic soda made from?
Sodium hydroxide flake is actually made from liquid caustic soda. This method uses cell membrane technology. The solid soda caustic produced in this method has the best quality and is free of contaminants such as heavy metals.
The product of this process is highly moisture absorbent and water-soluble and is used in different industries.
The purity of this product is at least 99%. Solid sodium hydroxide is usually packaged in polyethylene or polypropylene (PP / PE) bags with a standard weight of 25 kg.
We will explain more about packaging of the product in the following sections.
Sodium hydroxide (Caustic soda formula is NaOH) is also an inorganic (mineral) compound. This compound is based on and made from a solid metal, which is sodium. Sodium is solid, white, and highly caustic, and it is categorized among alkaline salts.
Solid caustic soda is usually available in flat pellets, flakes, granules and standard ready-made solutions with concentrations of 30% and 50% in different volumes.
Sodium hydroxide in the saturated solution forms a concentration of approximately 50% wt with water. Sodium hydroxide is soluble in water, ethanol and methanol. This alkaline substance easily absorbs moisture and carbon dioxide existing in the air.
Approximately the largest amount of caustic soda is produced by electrolysis of sodium chloride solution.
In the electrolysis method, one of the three types of electrochemical cells of mercury, diaphragm cell and membrane cells is used. The electrolysis process generally produces 2.25 tons of 50% caustic soda per ton of chlorine.
The raw material for producing solid caustic soda is common salt (sodium chloride), which is usually found in the form of underground sediments and is dissolved in a water source and pumped to the surface by high pressure.
Sodium chloride solution is called salt, which is the primary feed for producing caustic soda.
How to make caustic soda?
1- Mercury cell electrolysis
In the traditional method, electrolysis was conducted to produce solid caustic soda in mercury amalgam cells or diaphragm cells. Considering the electrolysis., the ion exchange membrane cell offers better environmental and economic benefits.
In the United States, the main method of sodium hydroxide production in electrolysis is through the diaphragm cell, while mercury and membrane cells are more typically used in Europe. Mercury cells gradually became obsolete due to environmental considerations and governments’ policies.
In the mercury cell, sodium and chloride ions are discharged as amalgam ions of sodium mercury and chlorine, respectively. The amalgam is transferred to a completely separate container (chamber), where it reacts with water.
Finally, a solution of sodium hydroxide and hydrogen gas is produced.
2- Diaphragm cell electrolysis method
The diaphragm cell is usually made of asbestos. In this cell, a salt water stream from the anode to the cathode occurs, separating the chlorine and hydrogen gas space through a diaphragm.
By discharging the hydrogen ions, the hydroxide ions are accumulated in the cathode chamber with dissolved sodium ions and produce sodium hydroxide (soda).
By the flow of liquid from one chamber to another, the reversible migration of hydroxide ions from the cathode to the anode is prevented.
3- Membrane cell electrolysis
In the membrane process, to produce Iranian caustic soda, the ion exchange membrane acts as a barrier against gas and liquid streams, allowing only sodium ions to pass through both chambers.
Sodium ions pass through the membrane hydrated, and sodium hydroxide is produced at the cathode from which hydrogen is released.
Chlorine gas is released at the anode. In this method, the membrane is a tetrafluoroethylene copolymer or a similar fluorine monomer.
You can see an example of these reactions in the following figure:

membrane cell electrolysis** A simple comparison between all three of the above methods in producing caustic soda:
Mercury cells are cheaper than diaphragm cells when the cost of using electricity to produce caustic soda is cheaper and produce highly concentrated and pure products, but mercury should be released as effluent.
Diaphragm cells need a lot of heat energy to concentrate the soda solution. If they have a cheaper structure, they can be even cheaper than mercury cells.
Using membrane cells to produce caustic soda is growing due to less initial capital and energy costs and not having environmental problems.
The product obtained by membrane cell method is purer. In this way, the membranes can pass different flows in the solution and carry out the production process at a lower cost of electricity consumption.
4- An overview of the chlor-alkali process

Caustic Soda production by chlor-alkali methodSolid sodium hydroxide is produced industrially as a 50% solution due to chemical changes in the chlor-alkali process. In this process, chlorine gas is also produced. Solid sodium hydroxide is obtained by evaporating water from this solution.
The reaction of producing caustic soda with alkaline sodium carbonate solution can be seen in the following section (this method was used in the past):
Ca(OH)2+Na2CO3 → CaCO3+NaOH
The above process was replaced by the Solvay process in the late 19th century. The solo process was in turn replaced by the chlor-alkali process that is used today in the industry to produce caustic soda.
Physical and chemical properties of caustic soda
| Compound names | |
| Naming in IUPAC Sodium hydroxide |
|
| Other typical names Caustic soda Lye Ascarite White caustic Sodium hydrate |
|
| Identification index | |
| 1310-73-2 | CAS Number |
| 215-185-5 | EC Number |
| 14798 | PubChem CID |
| 1824, 1823 | UN number |
| Physical and chemical properties | |
| NaOH | Chemical formula |
| 39.9971 g mol−1 | Molar mass |
| White, waxy, opaque crystals | Appearance |
| Odorless | Odor |
| 2.13 g/cm3 | Density |
| 323 °C (613 °F; 596 K) | Melting point |
| 1,388 °C (2,530 °F; 1,661 K) | Boiling point |
|
418 g/L (0 °C) |
Solubility in water |
|
Solubility in other solvents |
| 238 g/L | Solubility in Methanol |
| <<139 g/L | Solubility in Ethanol |
| <2.4 kPa (at 20 °C) | Steam pressure |
| -0.56 (NaOH(aq) = Na+ + OH–) | Open separation constant (pKb) |
| Structural features | |
| Orthorhombic, oS8 | Crystal structure |
| Thermochemical properties | |
| 59.5 J/mol K | Thermal capacity (C) |
| 64.4 J·mol−1·K−1 | Standard molar entropy (So298) |
| −425.8 kJ·mol−1 | Standard enthalpy of formation (ΔfH⦵298) |
| −379.7 kJ/mol | Gibbs free energy (ΔfG˚) |
| Sodium hydroxide related compounds | |
| Sodium hydrosulfide Sodium hydride |
Anions |
| Caesium hydroxide Lithium hydroxide Potassium hydroxide Rubidium hydroxide Francium hydroxide |
Cations |
Caustic soda solubility
Caustic soda solubility in 0 °C water is 418 g/l, in 25 °C water is 1000 g/l, and in 100 °C is 3370 g/l. It is also soluble in glycerol and ammonia. It is not soluble in ether.
Last Seen













 Top 10 caustic soda flakes suppliers in Iran
Top 10 caustic soda flakes suppliers in Iran


Users Comment
I am Ali Shojaie
We need a caustic soda production line with a capacity of 10 tons per day from the electrolysis process of water and salt using the membrane cell method with complete installation and operation. Please take appropriate action in this regard.
Thanks
How Urea Can Hydrate and Exfoliate Your Skin
Sodium carbonate| Soda ash; features and applications
Copper Sulfate; Features and applications
Sulfur; Features and applications
Zinc sulfate; Features and applications
Urea; Features and applications
Sodium bicarbonate; Features and applications
Ammonium Sulfate; Features and applications
What is the use of caustic soda? An Overview of the caustic soda uses
Analysis of September manufacturing trends in Asia
The impact of growth in major Asian economies on petrochemical demand
Aramco loan $2 billion to Petro Rabigh to cover cash shortfall
16Th International ArabPlast 2023
International Iran Conmine 2023
International Iran Nano 2023
17Th International Iranplast Fair 2023
12th International Composite Expo 2023
International IFarm Agro Fair 2023
22Nd International Paint Resin Coatings Fair
Sodium carbonate| Soda ash; features and applications
Copper Sulfate; Features and applications
Sulfur; Features and applications
Zinc sulfate; Features and applications
Urea; Features and applications