Sodium carbonate| Soda ash; features and applications

Sodium carbonate, Na2Co3, (=also known as washing soda, soda ash, and soda crystals) is a mineral compound that has different hydrated structures. All forms of this compound include white, odorless, water-soluble salts that produce moderately alkaline solutions in water. Historically, this substance was extracted from the ashes of plants grown in sodium-rich soils.
Contents
A Look at the History of sodium carbonate
Humans have known and used soda ash for thousands of years. The ancient Egyptians extracted this compound from a mineral called Natron, which was found at the bottom of dry lakes. Natron is a combination of soda ash and baking soda. Egyptians used its soda in embalming corpses. This combination kept the bodies of the dead dry and prevented them from decaying. This technique was so effective that some mummified bodies over 3000 years old are still in the same condition as when they died. Over the centuries, sodium carbonate was also produced from the combustion of organic matter, especially seaweed. In this production method, this common compound is soda ash.
Burning dead plants does not produce very large amounts of sodium carbonate, so chemists of the time looked for synthetic methods to produce this important compound. The first breakthrough in this quest came in 1791 when the French chemist Nicolas LeBlanc (1806-1742) devised a method for producing sodium carbonate that became an industry standard for nearly a century.
In 1900, almost all sodium carbonate produced in the world was made by the Solvay process. In the following, we explain these processes.
Soda ash production methods
Production of sodium carbonate from the mine
Trona, also known as trisodium hydrogen bicarbonate dihydrate, is mined in several regions of the United States and supplies nearly all of the region’s soda ash consumption. Large natural deposits discovered in 1938, such as those near Green River, Wyoming, made mining more economical than industrial production in North America. There are significant reserves of trona in Turkey. Two million tons of soda ash have been extracted from deposits near Ankara. Also, from some lakes such as Lake Magadi in Kenya, this compound is extracted by dredging operations. Hot springs produce lake salt continuously, provided the rate of dredging does not exceed the rate at which the spring draws water, a perfectly stable source of sodium carbonate.
Production of soda ash from plants and algae (Barilla and kelp)
Several “halophyte” (salt-tolerant) plant species and seaweed species can be processed to produce the crude form of sodium carbonate; These sources were mostly consumed in Europe and other places until the early 19th century. Plants or seaweed were dried and burned. Then the ashes were washed with water to form an alkaline solution. This solution was boiled dry to produce the final product, which was called “soda ash”. “Barilla” is a trade term for the crude form of potash obtained from seaweed, or kelp.
LeBlanc process for the production of sodium carbonate
In 1792, French chemist Nicolas LeBlanc patented a process for producing sodium carbonate from salt, sulfuric acid, limestone, and coal. In the first step, sodium chloride is treated with sulfuric acid in the Mannheim process. This reaction leads to the production of sodium sulfate (salt cake) and hydrogen chloride:
2NaCl + H2SO4 → Na2SO4 + 2HCl
Salt cake and crushed limestone (=calcium carbonate) are reduced under heating with coal. This conversion consists of two parts. The first is the carbothermic reaction in which coal, the carbon source, reduces sulfate to sulfide:
Na2SO4 + 2C → Na2S + 2CO2
The second stage of the reaction is the production of sodium carbonate and calcium sulfide:
Na2S + CaCO3 → Na2CO3 + CaS
This mixture is called black ash. Soda ash is extracted from black ash with water. From the evaporation of this extract, solid sodium carbonate is obtained.
The hydrochloric acid produced by the Leblanc process was a major source of air pollution, and the calcium sulfide byproduct also caused waste disposal problems. However, this basic method of producing sodium carbonate remained in place until the late 1880s.
Production of sodium carbonate in the Solvay process
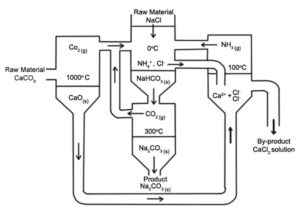
In 1861, Belgian chemist Ernest Solvay devised a method for producing sodium carbonate by first reacting sodium chloride, ammonia, water, and carbon dioxide to produce sodium bicarbonate and ammonium chloride:
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
Then the resulting sodium bicarbonate was converted into sodium carbonate by heating and water and carbon dioxide were released:
2NaHCO3 → Na2CO3 + H2O + CO2
Ammonia was produced from the by-product of ammonium chloride and by treating it with lime (calcium oxide) remaining from the production of carbon dioxide:
2NH4Cl + CaO → 2NH3 + CaCl2 + H2O
The Solvay process recovers ammonia. Only salt water and limestone are used in this process and calcium chloride is the only waste product. This process is considerably more cost-effective than the Leblanc process, which produces two waste products, calcium sulfide, and hydrogen chloride.
The Solvay process quickly dominated sodium carbonate production worldwide. By 1900, 90% of this material was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.
Production of sodium carbonate in the Hou process
This process was developed by the Chinese chemist Hou Debang in the 1930s. Carbon dioxide, the primary byproduct, was pumped through a saturated solution of sodium chloride and ammonia to produce sodium bicarbonate by these reactions:
CH4 + 2H2O → CO2 + 4H2
3H2 + N2 → 2NH3
NH3 + CO2 + H2O → NH4HCO3
NH4HCO3 + NaCl → NH4Cl + NaHCO3
baking soda Because of its low solubility is collected as a precipitate and then heated to about 80 °C (176 °F) or 95 °C (203 °F) to obtain pure sodium carbonate, similar to the last step of the Solvay process.
More sodium chloride is added to the remaining ammonium and sodium chloride solution. Also, more ammonia is pumped into this solution at 30-40 degrees Celsius. Next, the temperature of the solution drops below 10 degrees Celsius. The solubility of ammonium chloride is higher at 30°C than sodium chloride and lower at 10°C. Due to this temperature-dependent solubility difference and common ion effect, ammonium chloride precipitates in sodium chloride solution.
Hou’s process is more economical because it does not require more ammonia and, on the other hand, the ammonium chloride byproduct can be sold as fertilizer.
Properties and applications of sodium carbonate
The physical and chemical properties and characteristics of this powdery solid composition make it widely used in various industries. In full, we discuss these features in the table in the next section.
Physical and chemical characteristics of soda ash
| Sodium carbonate | |
| compound names | |
| nomenclature Sodium carbonate |
|
| Name suggested by IUPAC Disodium carbonate |
|
| Other common names Soda ash washing soda Soda crystal Sodium trioxocarbonate |
|
| indicator species | |
| CAS Number | 497-19-8 (without water) 5968-11-6 (single water) 6132-02-1 (ten water) |
| EC Number | 207-838-8 |
| E number | E500(i) |
| PubChem CID | 10340 |
| RTECS number | VZ4050000 |
| properties and characteristics | |
| Chemical formula | Na2CO3 |
| Molar mass | 105.9888 g/mol (without water) 286.1416 g/mol (ten water) |
| Appearance | White solid with moisture absorption properties |
| Smell | Odorless |
| Density | 2.54 g/cm3 (25 °C, without water) 1.92 g/cm3 (856 °C) 2.25 g/cm3 (single water) 1.51 g/cm3 (seven waters) 1.46 g/cm3 (ten water) |
| Melting point | 851 °C (1,564 °F; 1,124 K) (single water) 100 °C (212 °F; 373 K) decomposes (single water) 33.5 °C (92.3 °F; 306.6 K) decomposes (seven waters) 34 °C (93 °F; 307 K) (ten water) |
| Solubility in water | without water, g/100 mL: 7 (0 °C) 16.4 (15 °C) 34.07 (27.8 °C) 48.69 (34.8 °C) 48.1 (41.9 °C) 45.62 (60 °C) 43.6 (100 °C) |
| Solubility in other solvents | Soluble in alkalis and glycerol Relatively soluble in alcohol insoluble in carbon disulfide; acetone; Alkyl acetate; alcohol; benzonitrile; Liquid ammonia |
| Solubility in glycerin | 98.3 g/100 g (155 °C) |
| Solubility in ethanediol | 3.46 g/100 g (20 °C) |
| Solubility in dimethylformaldehyde | 0.5 g/kg |
| Acidity (pKa) | 10.33 |
| Viscosity | 3.4 cP (887 °C) |
| Thermochemistry | |
| Heat capacity (C) | 112.3 J/mol·K |
| Molar standard entropy (S⦵298) | 135 J/mol·K |
| Standard enthalpy of formation (ΔfH⦵298) | −1130.7 kJ/mol |
| Gibbs free energy (ΔfG⦵) | −1044.4 kJ/mol |
| Related compounds | |
| corresponding anions | Sodium bicarbonate |
| corresponding cations | Lithium carbonate Potassium carbonate Rubidium carbonate Caesium carbonate |
| Other related compounds | Sodium sesquicarbonate Sodium percarbonate |
read more: Caustic soda flakes and the methods of production
Uses of soda ash
Glass manufacturing industry
The sodium carbonate acts as a flux for the silica (2SiO, with a melting point of 1713 °C) and raises the melting point of the mixture to a level that can be achieved without the need for special materials. This “soda mix” dissolves slowly in water, so some calcium carbonate is added to the molten mixture to make the glass insoluble. Bottle and window glasses are made by melting such mixtures of sodium carbonate, calcium carbonate and silica sand and silicon dioxide.
As a water softener
Hard water contains dissolved compounds, usually calcium or magnesium compounds. Sodium carbonate is used to remove temporary and permanent hardness of water.
Since this substance is soluble in water and magnesium carbonate and calcium carbonate are insoluble, it is used to soften water by removing 2+ Mg and 2+ Ca. These ions form insoluble solid deposits after treatment with carbonate ions. Water becomes soft because it does not contain dissolved calcium ions and magnesium ions.
Food additives and cooking
Sodium carbonate has a variety of uses in cooking, mainly because it is stronger than baking soda but weaker than metal hydroxide alkalia (which may refer to sodium hydroxide or potassium hydroxide). Alkaline affects the production of gluten in the cooked dough. A solution of alkaline salts is used to taste Japanese noodles.
Bakers similarly use its soda ash as a replacement for freshwater to create special texture in cakes and improve the cake browning.
It is used in the production of syrup powder. The feeling of coolness and tingling of the mouth, due to the heat reaction between sodium carbonate and poor acid, is usually citric acid that releases carbon dioxide gas, and occurs when the syrup is moistened by saliva.
It is also used in the food industry as a food additive (code E500) as an acidity regulator, anticoagulant, volume agent and stabilizer.
Other soda ash uses:
- It is used as a relatively strong base in various industries. As a regular alkaline, it is preferred in many chemical processes because it is cheaper than sodium hydroxide and is much safer. In particular, the mildness of the alkalinity of this compound recommends its use in-home use.
- Sodium carbonate is used as a pH regulator to maintain sustainable alkaline conditions to produce photography film.
- Soda ash is a common additive in swimming pools and aquarium water to maintain the desired pH and carbonate hardness (KH).
- Sodium carbonate is used in dyeing fiber-specific dyes to ensure the appropriate chemical bonding of the color with cellulose fibers and usually before staining.
- In addition to CAO and other mild game compounds, sodium carbonate in the Froth flotation process is used to maintain the desired pH as a floating softener.
read more: uses of caustic soda
Safety
- This substance is non-flammable.
- The hypertensive temperature of the compound is not available.
- Sodium carbonate flammability points are not available.
- Flammable restrictions are not available.
- When sodium carbonate is heated to decompose, the NA2O smoke releases.
- The risks of fire are not available in the presence of various materials.
- The risks of explosion are not available in the presence of various materials.
- Sodium carbonate in contact with fluoride can be ignited and burn severely.
- Soda ash is decomposed in contact with fluorine at normal temperatures due to radiation.
- Soda ash responds with hot aluminum metal.
- This compound is stable.
- The unstable temperature is not available.
- Its unstable conditions are in the presence of incompatible substances such as acids and moisture.
- This material with phosphorus pentoxide, lithium, fluorine, fluoride, ammonia + silver nitrate, 6,4,2-tlic nitrotoluine, ammonia, acids, sodium sulfide + water, hydrogen peroxide, red metal aluminum, sodium sulfide, sodium sulfide It is incompatible.
- Sodium carbonate is decomposed in the presence of acids by pimples.
- Sodium carbonate concentrated solutions have mild corrosive properties for steel.
- Polymerization does not occur in this substance.
Storage and maintenance
Do not breathe dust when working with this compound. Wear the right protective clothing. Have good respiratory equipment, mask, glasses and gloves. Avoid contact with the chemical composition with the skin and eyes.
Sodium carbonate packages should be kept away from incompatible materials such as acids. Keep the material in the closed lid container. Keep the dishes and bags in a cool, well -ventilated place. This compound should not be stored at high temperatures; The best temperature is 24 ° C.
Packing

Light sodium carbonate packaging and dence carbonate sodium carbonate is available in the market with a weighted 50 kg. For major orders, the side bags are also available to the customer in a weight of 1000 kg.
Sodium carbonate manufacturers
Given the widespread use of this material in small and large industries, the demand for the purchase and sale of this important industrial material is significant. You can refer to the list of Shimico products for the purchase of Maragheh sodium carbonate, Semnan sodium carbonate and other petrochemical or soda ash factories.
Sources:
Last Seen
















Users Comment
How Urea Can Hydrate and Exfoliate Your Skin
Copper Sulfate; Features and applications
Sulfur; Features and applications
Zinc sulfate; Features and applications
Urea; Features and applications
Sodium bicarbonate; Features and applications
Ammonium Sulfate; Features and applications
What is the use of caustic soda? An Overview of the caustic soda uses
Caustic soda flakes and the methods of production
Analysis of September manufacturing trends in Asia
The impact of growth in major Asian economies on petrochemical demand
Aramco loan $2 billion to Petro Rabigh to cover cash shortfall
16Th International ArabPlast 2023
International Iran Conmine 2023
International Iran Nano 2023
17Th International Iranplast Fair 2023
12th International Composite Expo 2023
International IFarm Agro Fair 2023
22Nd International Paint Resin Coatings Fair
Copper Sulfate; Features and applications
Sulfur; Features and applications
Zinc sulfate; Features and applications
Urea; Features and applications
Sodium bicarbonate; Features and applications