Copper Sulfate; Features and applications

Copper sulfate along with its characteristics, production method and applications
Copper Sulfate(II) It is an inorganic compound with the chemical formula CuSO4.x(H2O), where x can vary from 0 to 5. Copper sulfate pentahydrate (x=5), is the most common form of this compound. The old names of this compound include blue copperas (blue alum), blue vitriol, blue stone, copper vitriol and Roman vitriol.
CuSO4.5(H2O) pentahydrate, which is the most common salt structure in this form, is a light blue solid. This salt dissolves in water as an exothermic reaction and turns into an almost complex compound with the structure +2[Cu(H2O)6]. This compound has an octahedral molecular geometry.
The solid pentahydrate structure shows a polymer structure in which copper is bound to four water ligands. Anhydrous copper sulfate is a light gray powder.
Contents
A look at the history of copper sulfate
What is copper sulfate and how was it first used? It has been matter used in the United States since the 1700s and was first registered for use in the United States in 1956. The US EPA completed the acceptance and registration process for copper sulfate in 2009. This combination has been used in the dyeing and electroplating industry for years and now it covers a wide range.

How is copper sulfate produced?
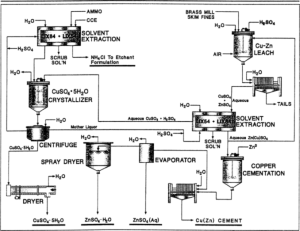
In order to better understand what copper sulfate is, it is necessary to get acquainted with the production method of this substance. This material is industrially produced by refining copper metal with hot concentrated sulfuric acid or its oxides with dilute sulfuric acid. For laboratory use, lab grade copper(II) sulfate is usually purchased. Copper sulfate can also be produced by slowly leaching copper ore. Some bacteria may be used to speed up this process.
Commercial blue copperas is usually about 98% pure copper sulfate and may contain small amounts of water. Anhydrous copper In terms of crime (II) sulfate is 39.81% copper and 60.19% sulfate by mass.
If this compound is blue in color and in aqueous form, it contains 25.47% copper, 38.47% sulfate (12.82% sulfur), and 36.06% water by mass.
Four types of sizes for crystals of this mineral compound have been introduced based on its application: large crystals (40-10 mm), small crystals (2-10 mm), fine snow-like crystals (less than 2 mm) and powder. Copper sulfate (less than 0.15 mm).
Properties of copper sulfate
Copper(II) sulfate pentahydrate decomposes before melting. When heated to 63°C (145°F), it loses two molecules of water, then two more at 109°C (228°F), and the last water molecule at 200°C (392°F) Disappears.
At 650°C (1,202°F), copper(II) sulfate decomposes into copper(II) oxide (CuO) and sulfur trioxide (3SO).
Copper sulfate reacts with concentrated hydrochloric acid to produce tetrachlorocuprate (II):
Cu2+ + 4 Cl− → CuCl42−
Physical and chemical characteristics
| Name of the compound | |
| IUPAC Copper(II) sulfate |
|
| Common names Cupric sulphate Blue vitriol (pentahydrate) Bluestone (pentahydrate) Bonattite (trihydrate mineral) Boothite (heptahydrate mineral) Chalcanthite (pentahydrate mineral) Chalcocyanite (mineral) Copper Sulphate pentahydrate |
|
| Identifier indicators | |
| CAS Number |
|
| EC Number | 231-847-6 |
| E number | E519 |
| PubChem CID | 24462 |
| Features and properties | |
| chemical formula | CuSO4 (Anhydrous form) CuSO4·5H2O (Form 5 of water) |
| Molar mass | 159.60 g/mol (Anhydrous form) 249.685 g/mol (Form 5 of water) |
| Appearance | White-gray (Anhydrous form) blue (Form 5 of water) |
| density | 3.60 g/cm3 (Anhydrous form) 2.286 g/cm3 (Form 5 of water) |
| melting point | Aqueous copper sulfate form 5 decomposes at 110 °C The anhydrous form of copper sulfate decomposes at <560 °C |
| boiling point | decomposes |
|
Solubility in water |
1.055 Molal (10 °C) 1.26 Molal (20 °C) 1.502 Molal (30 °C) |
| Solubility in other solvents | The anhydrous form is soluble in ethanol The pentahydrate form is soluble at 10.4 g/L (18 °C) in methanol It is insoluble in ethanol and does not dissolve in acetone |
| structural features | |
| Crystal structure | Chalcocyanite orthorhombic (anhydrous form) Tri Clinic (Form 5 Water) |
| Thermochemical properties | |
| standard entropy Molar (So298) | 5 J K−1 mol−1 |
| Standard enthalpy formation (ΔfH⦵298) | −769.98 kJ/mol |
| flash point | unflamable |
| Related compounds | |
| corresponding cations | Iron (II) sulfate Manganese(II) sulfate Nickel(II) sulfate Zinc sulfate |
What are the uses of copper sulfate?
1.agriculture
This chemical is used in agriculture as a micronutrient. This substance causes the production of vitamin A and has a metabolic role. It also increases the biosynthesis and ripening of fruits. Copper has a much higher absorption through sulfate compounds because it can be absorbed in the form of Cu2 + cation. It is very suitable to use this material as a solution in water to compensate for the lack of copper in plants. 5A copper sulfate without nitric acid is suitable for use as agricultural fertilizer.
2.Fungicides and herbicides
Copper sulfate 5 water or pentahydrate is used as a fungicide. However, some fungi have the ability to adapt to high levels of copper ions. A suspension containing compounds of copper sulfate and calcium hydroxide is used to control the fungus of grapes, melons and mulberry plants. This material is produced by mixing copper sulfate aqueous solution and lime suspension.
A mixture of copper sulfate and ammonium carbonate is used in horticulture to prevent damping of seedlings. This mixture is used as a herbicide with non-agricultural uses such as the control of invasive aquatic plants and the roots of plants that grow in the vicinity of water transmission pipes .
Copper sulfate is used in swimming pools as an agent to destroy algae. A dilute solution of copper sulfate is used to treat parasitic infections of aquarium fish and is also used to remove snails from the aquarium.
Remember that copper ions are very toxic to fish. Most species of algae can be controlled with very low concentrations of copper sulfate.
3.Chemical analyses
Copper sulfate is used to perform many chemical experiments. This substance is used in Fehling’s solution and Benedict’s solution to test the reduction of sugars; Reactions in which copper(II) sulfate reduces soluble sulfate to red copper(I) oxide, in a soluble state. Copper(II) sulfate is also used in Biuret reagent to test proteins.
In the flame test, the copper ions emit a deep green light as the compound burns in the flame. This green color is much more intense than when barium burns in a flame.
4.Synthesis of organic compounds
Copper sulfate is used on a limited level in the synthesis of some organic compounds. The anhydrous salt of copper sulfate is used as a water reducing agent for the formation and modification of acetal groups. The hydrated salt can be mixed with potassium permanganate to produce an oxidizing compound to convert primary alcohols.
5.Rayon production
The reaction of copper sulfate with ammonium hydroxide produces tetraamminecopper (II) sulfate or Schweizer’s reagent, which is used to dissolve cellulose in Rayon industrial production.
6.Applications of copper sulfate in industry
Copper sulfate, blue stone, blue vitriol or blue alum, is the best known and most useful example of copper salt. In fact, this material is often used as a raw material for the production of many other copper salts.
Today, there are more than 100 producers of copper sulfate in the world, and its global consumption is about 275,000 tons per year. It is estimated that approximately three-quarters of this copper salt is used in agriculture, mainly as a fungicide and also to treat copper-deficient soils.
Copper sulfate is a chemical substance with diverse properties and has wide applications in industry. In the past, this compound was mostly used in dyeing and electroplating, and today a large percentage of this substance is used for agricultural purposes.

We have listed the uses of copper sulfate in the industry as follows:
- Preparation of raw materials used in the synthetic fiber industry
- In the metal industry, large amounts of copper sulfate are used as an electrolyte in copper purification, to cover steel wire and wire before stretching the wires, and in various stages of copper plating.
- In the mining industry, blue alum copperas is used in different concentrations as an activator for flotation of lead, zinc, cobalt and gold ore.
- It is used for engraving in the printing industry.
- In the paint industry, copper sulfate is used in antifouling paints and plays an important role in glass coloring .
- Copper sulfate in agriculture for preparation of fungicide, production of poisons to destroy weeds, production of insecticide, control of fungal diseases, correction of copper deficiency in soil, correction of copper deficiency in animals, growth stimulant for fattening broilers, to eliminate Snails, soil disinfectant, are used to prevent diseases of tomato plants, etc.
- It is used in swimming pools to kill algae and microorganisms.
- It solution is also used to disinfect the environment of keeping calves, cows and sheep.
- It fertilizer is suitable for providing plant nutrients in rice fields. And it is also used to protect against algae growth in flower pots.
- In the adhesive industry, copper sulfate is used as a preservative, an additive for paste and book binding adhesives. This additive is added to silicate adhesives to make them water resistant.
- In the construction industry, copper sulfate is used to prepare other wood and timber preservatives, to prevent the growth of wood worms and wood decay. In the production of colored concrete for use in swimming pools and its surroundings, this compound is used both as a coloring agent and an antiseptic.
- It solution can prevent the growth of tree roots in sewage.
- The preparation of catalysts for use in many industries, the purification of gases, for example, the removal of hydrogen chloride and hydrogen sulfide, the purification of zinc sulfate solutions, are examples of the applications of this mineral salt in the chemical industry.
- The source of other copper compounds such as copper carbonate silicate / arsenite / aceto-arsenite / resinate / stearate / tartrate / chromate / chlorate / alginate / fluoride / hydroxide / chloride / cyanide and copper ammonium is from the blue alum copperas
- For painting glass, painting cement and plaster, painting ceramic dishes, changing metal colors, for example darkening of zinc, painting aluminum, reagent in the preparation of intermediate materials for the preparation of phthalocyanine dyes, all of the applications of copper sulfate in the paint and pigment industry are.
- In the leather industry, copper sulfate is used for dyeing, as a reagent in tanning processes.
- Copper sulfate solution, in the place of an electrolyte in copper refining, in copper plating, the manufacturer of electrodes and electrolytes in batteries, in the production of copper powder, in aluminum plating and anodizing, copper-coated wire, providing a suitable surface for iron and steel numbering is used .
- In the preparation of composite materials for printing ink, synthetic rubber, in the preparation of catalysts used in cracking some liquid and gaseous gases, in the preparation of copper chloride, in the purification of butadiene and the separation of acetylene derivatives, in the preparation of catalysts used in the chlorination of rubber latex, in the purification of petroleum oils, from This copper salt is used.
- The use of copper sulfate in the textile industry to prepare copper compounds for anti-rot fabrics and other fabrics, sandbags with anti-rot properties, in the copper ammonium process for the production of artificial silk, in the production of aniline and diazo black dyes for dyeing, to increase color stability In textiles, it is very well known.
- Other industrial applications of copper sulfate include improving the quality of coke burning, laboratory analysis, preparation of chlorophyll as a food coloring agent, production of green color in fireworks, activating agent in preparation of activated carbon, wood pulp preservative, production of fruit wrapping paper for They prevent rotting during storage.
read more: Sodium carbonate| Soda ash; features and applications
Safety of copper sulfate (MSDS)
Toxicity of copper sulfate to humans
This product is an irritant. Common ways a person can be exposed to copper sulfate toxicity are through eye or skin contact, as well as inhalation of dust and particulate matter. Skin contact may result in itching or eczema. Eye contact with copper sulfate can cause inflammation of the eyelid lining, ulcers and clouding of the cornea.
Copper sulfate is moderately toxic if accidentally ingested. According to studies, the lowest dose of copper sulfate that has a toxic effect on humans is 11 mg per kilogram. Due to its stimulating effect on the digestive system, vomiting symptoms are observed. Symptoms can be more severe if copper sulfate is retained in the stomach.
After swallowing 1-12 grams of copper sulfate, symptoms of poisoning may occur in the form of a metallic taste in the mouth, burning and pain in the chest, nausea, diarrhea, vomiting, headache, which leads to yellowing of the skin. In cases of copper sulfate poisoning, brain, stomach, liver, or kidney damage may also occur.
Toxicity of copper sulfate for the environment
Copper sulfate is very soluble in water and therefore it is easy to spread it in the environment. Copper is found in soil from industrial sources, motor vehicles and architectural materials.
Copper sulfate is mainly present in surface soil and tends to bond with organic materials and compounds. The more acidic the soil, the lower its binding ability. Too much copper can be toxic to plants because it inhibits photosynthesis.
Copper is non-toxic to honey bees but has been shown to be moderately toxic to birds. Studies with several aquatic species have shown that copper is very, very toxic to fish and aquatic animals. Salmon are particularly sensitive to copper.
Since this product is used to control algae in ponds and lakes, oxygen depletion has been cited as the most common cause of fish deaths. Even small concentrations of copper can be harmful to fish and aquatic life.
Maintenance
You should wash your hands thoroughly after working with the copper sulfate compound. Wash work clothes before reuse to remove contamination. Warehouses for storing bags of this composition must have proper ventilation.
Work with this material in such a way as to minimize the generation and accumulation of dust. Avoid contact with skin, eyes and clothing. Avoid breathing dust.
Solid copper sulfate should be stored in a tight container with a closed lid. Bags and packaging should be stored in a cool, dry and well-ventilated place away from incompatible materials. Protect the warehouse from increased volume of moisture.
Copper sulfate packaging

Copper sulfate packaging is common in 20 kg laminated and 25 kg bags with closed lids. 1000 kg bags are used to transport this composition in bulk. Plastic containers with a weight of 1 kg are usually available for buying and selling laboratory grade copper sulfate. The price of blue copperas is different in each of the agricultural, industrial and laboratory grades.
Market situation and buying and selling of copper sulfate fertilizer
It is possible to buy and sell copper sulfate in different packages in the market. A laboratory blue copperasis available for scientific and research laboratories. The industrial grade of this compound is used in various uses that we have mentioned above.
Shimico, a specialized authority for the sale of industrial chemicals, has provided you with the opportunity to buy copper sulfate online.
 Top 10 copper sulfate suppliers in Iran
Top 10 copper sulfate suppliers in Iran
Sources:
ttps://en.wikipedia.org/wiki/Copper(II)_sulfate
https://copperalliance.org.uk/about-copper/copper-compounds/uses-copper-sulphate/
Last Seen
















Users Comment
How Urea Can Hydrate and Exfoliate Your Skin
Sodium carbonate| Soda ash; features and applications
Sulfur; Features and applications
Zinc sulfate; Features and applications
Urea; Features and applications
Sodium bicarbonate; Features and applications
Ammonium Sulfate; Features and applications
What is the use of caustic soda? An Overview of the caustic soda uses
Caustic soda flakes and the methods of production
Analysis of September manufacturing trends in Asia
The impact of growth in major Asian economies on petrochemical demand
Aramco loan $2 billion to Petro Rabigh to cover cash shortfall
16Th International ArabPlast 2023
International Iran Conmine 2023
International Iran Nano 2023
17Th International Iranplast Fair 2023
12th International Composite Expo 2023
International IFarm Agro Fair 2023
22Nd International Paint Resin Coatings Fair
Sodium carbonate| Soda ash; features and applications
Sulfur; Features and applications
Zinc sulfate; Features and applications
Urea; Features and applications
Sodium bicarbonate; Features and applications